Chlorine: Preparation, Properties and Uses
Chlorine: Preparation, Properties and Uses: Overview
This Topic covers sub-topics such as Bleaching powder, Chlorine, Uses of Chlorine, Physical Properties of Chlorine, Preparation of Chlorine and, Chemical Properties of Chlorine
Important Questions on Chlorine: Preparation, Properties and Uses
The products obtained from the following chemical reactions are respectively
(i)
(ii)
Autoxidation of bleaching powder gives two products and . The correct options of and are
An aqueous solution of is treated with each of the following. In which case bromine will be liberated?
An inorganic salt when heated with concentrated , evolves a colourless pungent smelling gas but with concentrated and , evolves a coloured pungent smelling gas which bleaches moist litmus paper. The coloured gas is
Name the catalyst used in the following reaction.
The overall reaction corresponding to electrolysis of aqueous solution is
on reaction with cold and dilute gives and
on reaction with excess of gives
Deacon's process of manufacture of chlorine is represented by the equation
Reaction of with hot and conc. produces
Deacon's process is used for the manufacture of
Which explosive substance is obtained, when proportion of dichlorine gas is more in the reaction of dichlorine gas with ammonia gas?
Match List with List
| List (Chemical reaction) | List (Name of process) | ||
| Contact process | |||
| Ostwald's process | |||
| Deacon's process | |||
| Haber's proces | |||
What is the colour of chlorine gas.
What is the name of the given reaction.
The gas formed when concentrated is added to a mixture of and is -
When chlorine is treated with sulphur, the product form will be:
Bleaching action of chlorine is by:
Which will not give gas ?
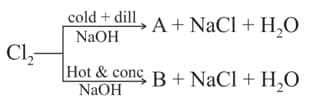
Compound & respectively are :
